- HOME
- Technical Info
Technical Info


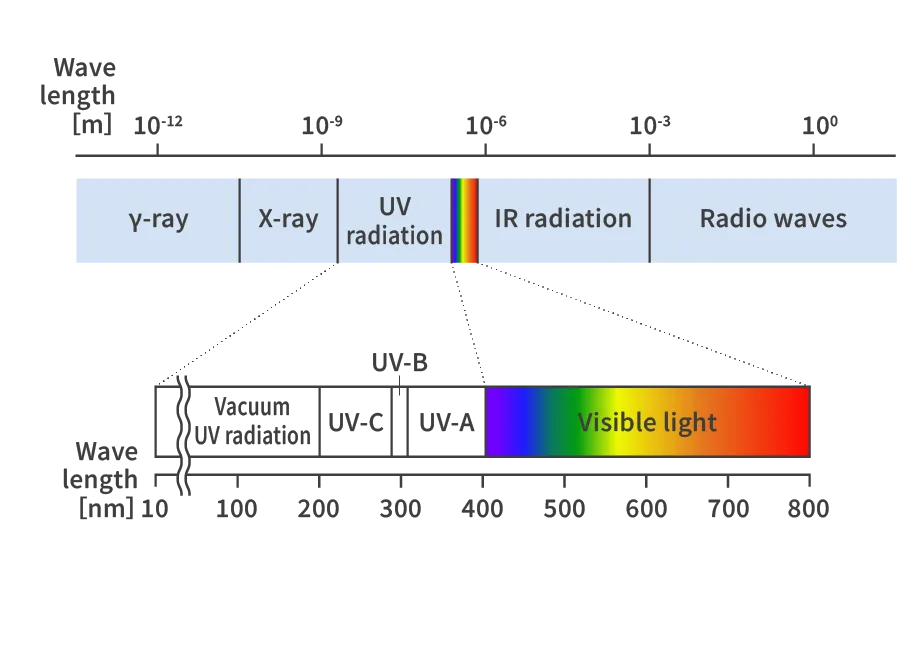
The light (electromagnetic waves) emitted from the sun showers a large amount of energy on our planet. There are various types of electromagnetic waves from the sun, including visible light, gamma rays, X-rays, UV radiation, IR radiation, and radio waves. UV radiation refers to light with wavelengths in the range of 10 to 400 nm and is classified from the shortest wavelength to the longest as Vacuum UV radiation, UV-C, UV-B, and UV-A. Depending on the wavelength, UV radiation can produce ozone, disinfect, and cause photochemical reactions.
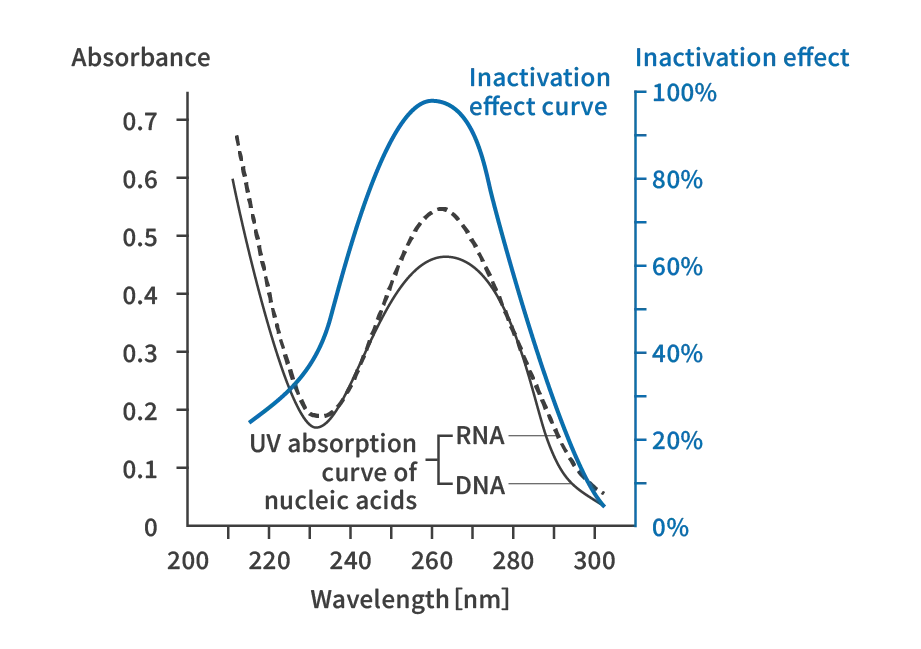
UV radiation changes parts of the nucleic acids (DNA, RNA) in microorganisms, stopping their ability to reproduce. This process is generally referred to as disinfection, but technically it is called inactivation. Figure shows the absorbance of nucleic acids against UV wavelengths. We can see that nucleic acids absorb at wavelengths around 260 nm. The same figure also shows the inactivation curve for microorganisms. We can see that the peak of this curve is also around 260 nm. In other words, the principle of UV disinfection is that nucleic acids absorb UV radiation around 260 nm, which causes a photoreaction to proceed and inactivates the microorganisms
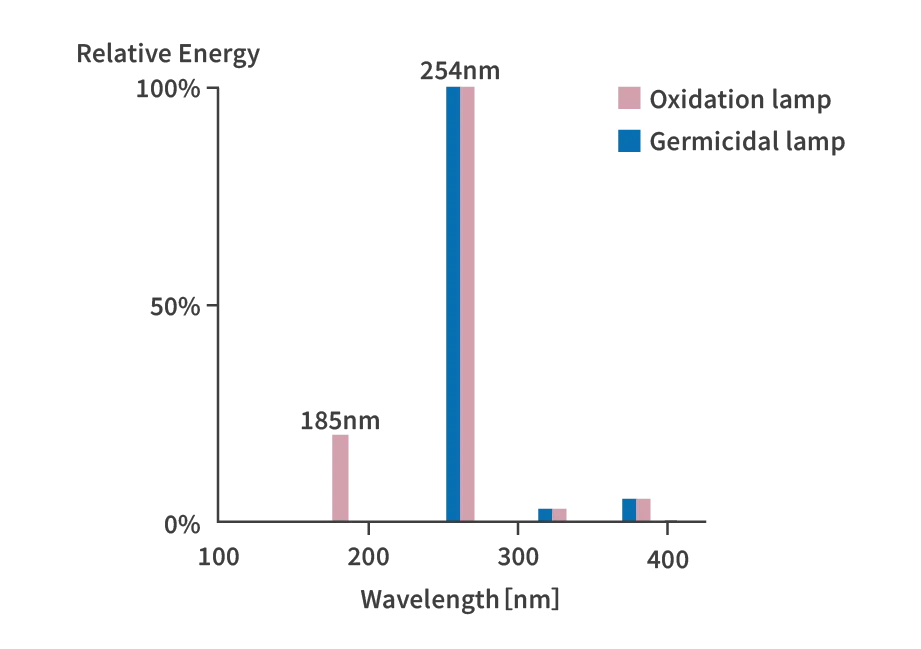
Organic matter can be efficiently decomposed and removed using a UV radiation source. One such light source is a low-pressure mercury lamp that emits Vacuum UV radiation with a wavelength of 185 nm. To generate OH radicals from water, it is necessary to dissociate the H-O bond in the water molecule H-O-H. The energy required for dissociation and the wavelength required for light absorption are 200 nm or less, so Vacuum UV radiation of 185 nm is used. To distinguish it from a germicidal lamp, it is also called an oxidation lamp.
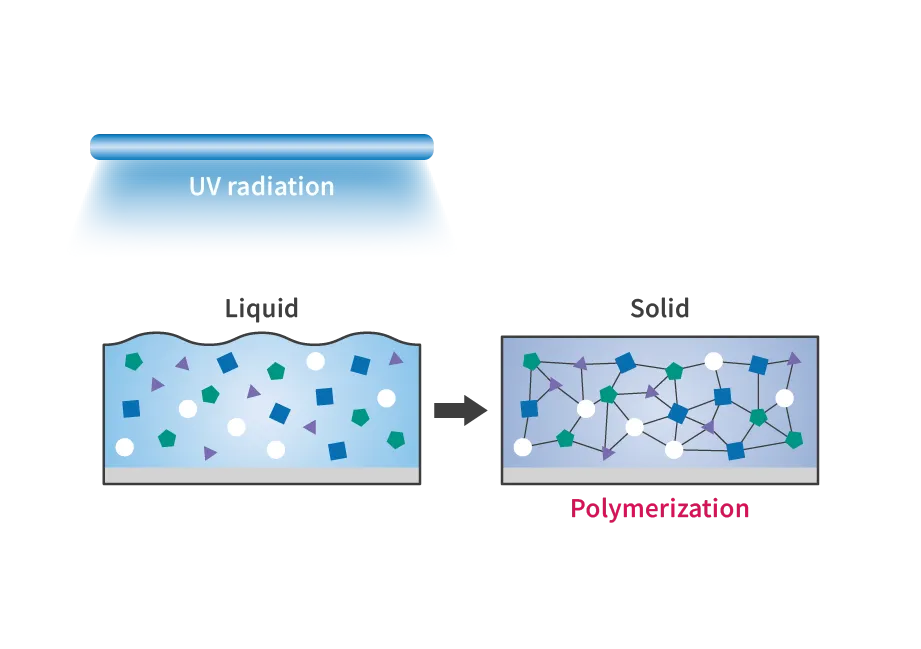
The curing of resin by UV radiation is a photochemical reaction process called “photopolymerization.” This technology uses UV energy to chemically change liquids into solids (monomers into polymers). Photocurable resins harden by absorbing light energy mainly in the UV range of wavelengths 250-400 nm. UV radiation with wavelengths of 365 nm reaches the inside of photocurable resins better than 254 nm, so UV radiation with wavelengths of 365 nm is generally used.
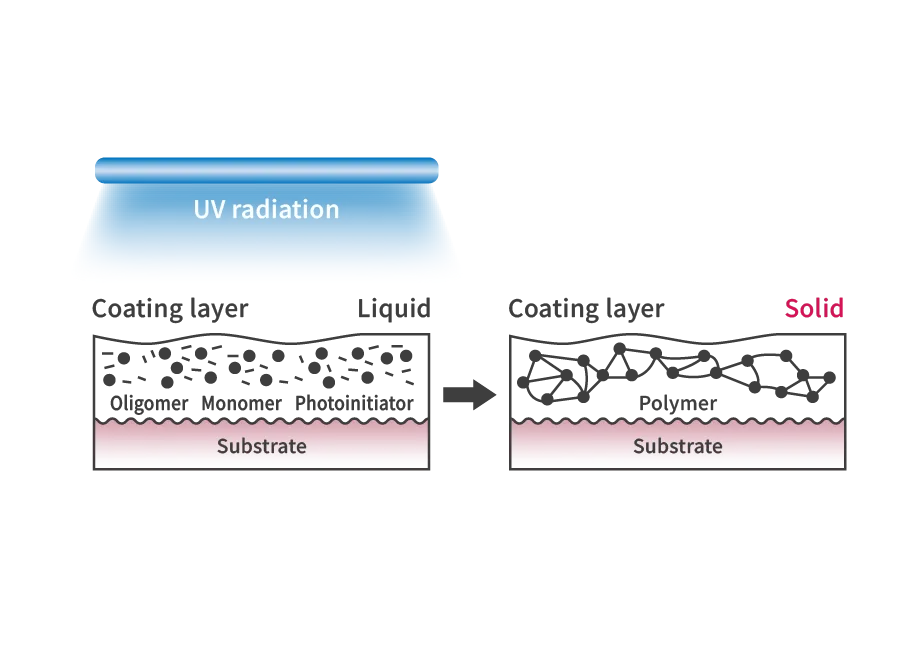
Adhesive bonding by UV radiation refers to bonding using a special “UV-curing resin” that hardens when exposed to UV radiation. The principle is that a chemical reaction called “photopolymerization” occurs in response to UV radiation, causing substances with small molecular weights (monomers) to bond and form substances with large molecular weights (polymers). As a result, the melting point of the material rises, causing the liquid to turn into a solid and bond (harden). In this case, the same 365 nm UV radiation is used as for resin hardening.
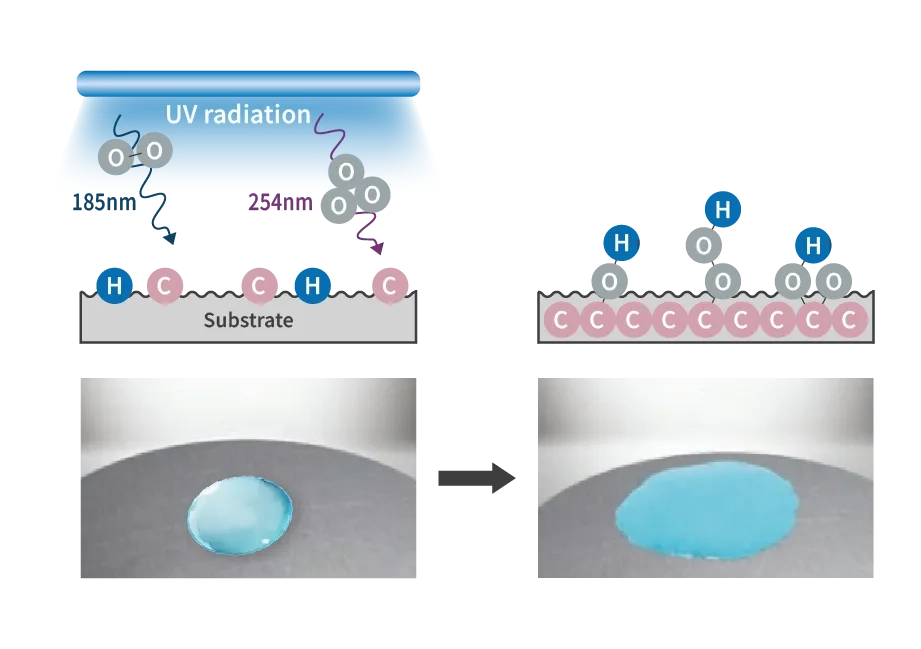
Active oxygen generated by UV radiation collides with the substrate surface, cutting the molecular chains of the surface layer, and reacts with the cut molecules to generate new functional groups (OH, CHO, COOH, etc.). These functional groups are highly hydrophilic and compatible with paints, adhesives, coating materials, etc., dramatically improving and enhancing adhesion. Low-pressure mercury lamps that efficiently radiate wavelengths of 185 nm and 254 nm are generally used as light sources for UV cleaning and modification equipment. The shorter the wavelength, the higher the energy, and wavelengths of 185 nm and 254 nm can cut the bonds of many organic substances.
Low-pressure mercury lamps are discharge lamps designed to have a mercury vapor pressure of around 1 Pa during steady operation to most efficiently obtain UV radiation with wavelengths of 254 nm or 185 nm, which are the resonance lines of mercury atoms. A noble gas, such as argon, at a pressure of several Torr is sealed inside as the starting gas. Lamps for disinfection use special glass that can transmit UV radiation with a wavelength of 254 nm, while lamps for surface treatment use quartz glass that can transmit Vacuum UV radiation with a wavelength of 185 nm. The optimal vapor pressure for the 185 nm resonance line is slightly higher than the mercury vapor pressure for the 254 nm resonance line, allowing for high output accordingly.
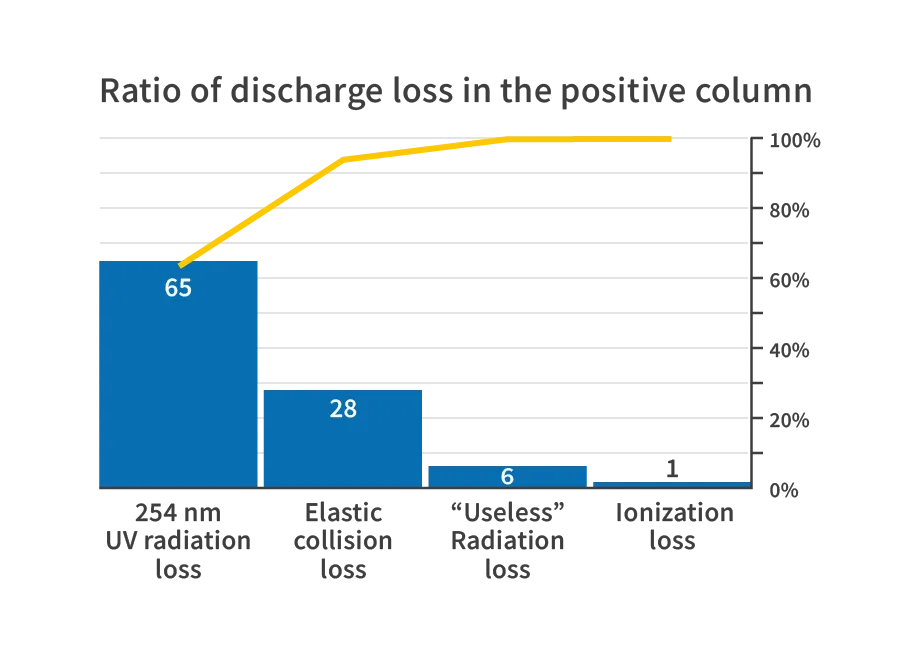
Low-pressure mercury lamps are considered the most efficient UV radiation source among all discharge lamps. Most of the energy from the electrons in the plasma is used to excite mercury atoms. The ionized mercury creates new electron-ion pairs, resulting in the efficient generation of UV radiation at 254 nm. Approximately 65% of the energy input into the positive column plasma of a UV lamp is converted into UV radiation at 254 nm. This conversion efficiency is the highest among discharge lamps, making it the most efficient light source.
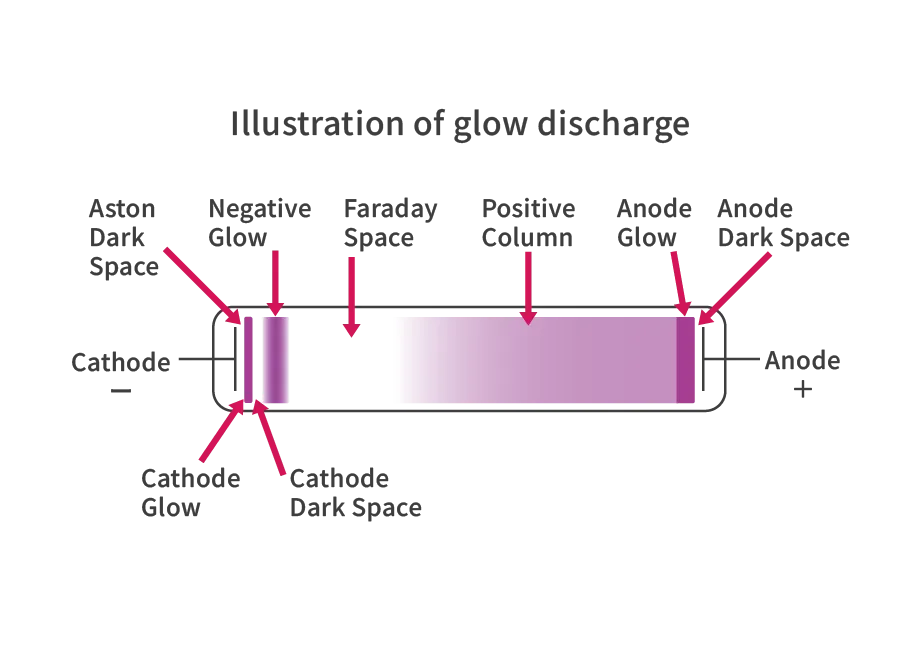
At the start of lighting the lamp, current is passed through the electrodes to preheat them, and the hot emitter (electron-emitting material) emits a large amount of thermoelectrons. The emitted thermoelectrons are accelerated by the electric field and are attracted to the opposite electrode, starting the discharge. The electrons flowing due to the discharge collide with mercury atoms sealed in the glass tube. The mercury atoms are excited and emit UV radiation when they release energy. Germicidal lamps and oxidation lamps use UV radiation as it is, but black lights and UVB lamps convert the UV radiation emitted from mercury atoms into longer wavelength UV radiation using phosphors and emit it outside the lamp.
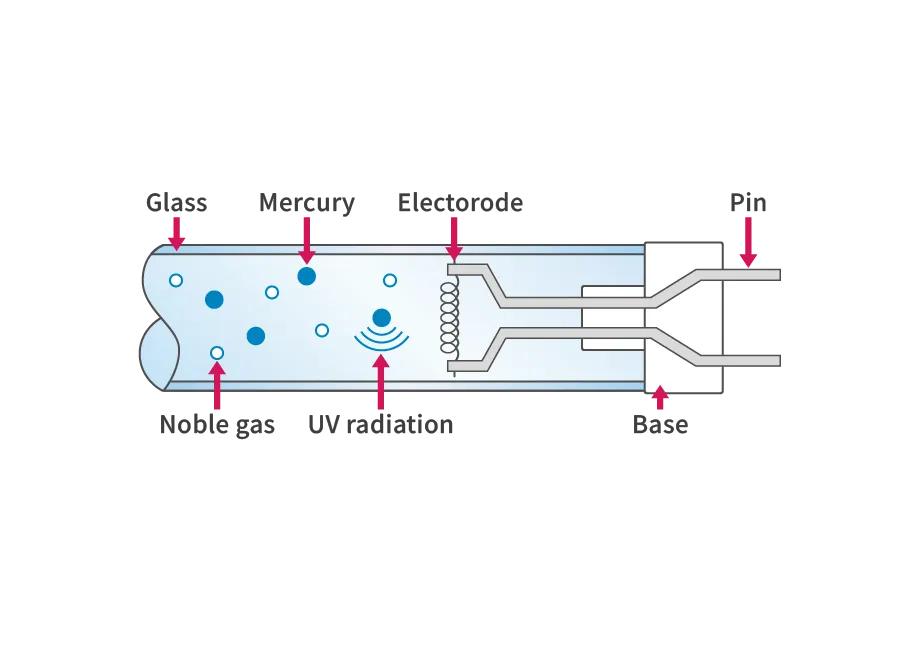
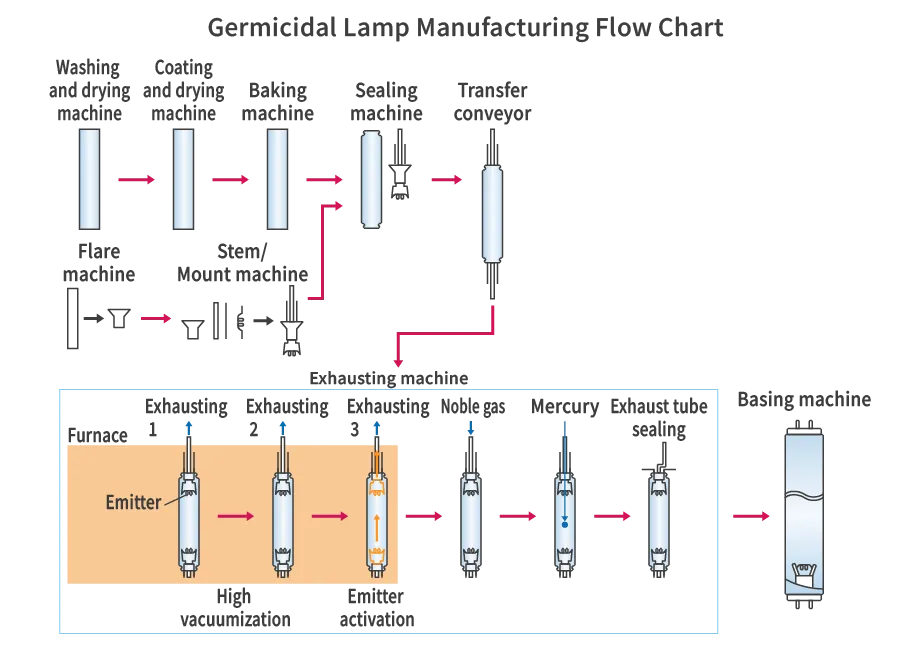
One pair of electrode structures, called stems, are sealed to both ends of a straight glass tube that transmits UV radiation using the flame of a gas burner. The electrodes are coiled filaments coated with an electron-emitting material (emitter). After exhausting the air through the thin tube of one of the stems and creating a vacuum, noble gas and a small amount of mercury are sealed into the glass tube, and the thin tube is tipped off to complete the exhaust. Finally, bases are placed on both ends of the glass tube and fixed with cement to complete the process.
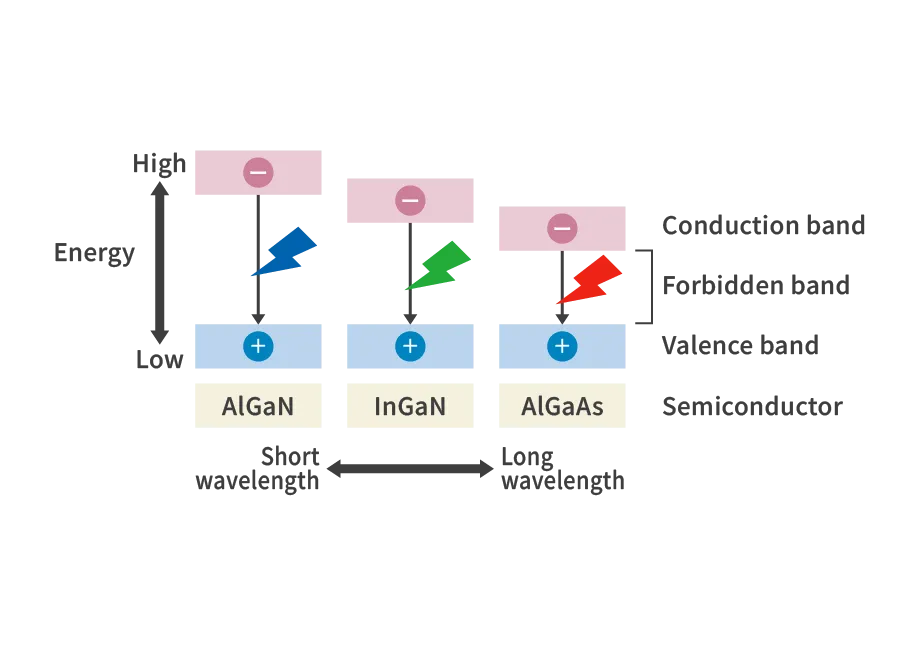
The diagram below explains how the emitted wavelength varies depending on the semiconductor material. The combination of holes and electrons at the p-n junction occurs when electrons fall from the high-energy conduction band to the low-energy valence band. The greater this energy difference, the higher the energy of the emitted light, i.e., the shorter the wavelength. The energy difference (forbidden band width) varies depending on the semiconductor material, so light-emitting diodes (LEDs) are made by selecting a material with a forbidden band that matches the desired emission color.
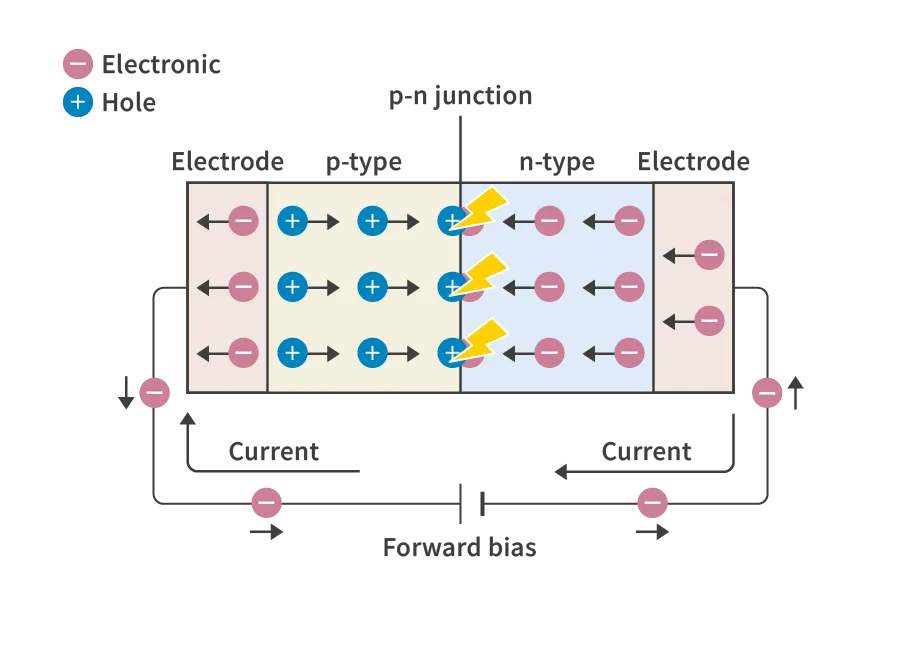
The basic principle of a light-emitting diode (LED) is illustrated in the diagram. A p-n junction is formed by joining a p-type semiconductor (which has many holes) and an n-type semiconductor (which has many electrons). When a forward voltage is applied to this element, holes and electrons move toward the p-n junction, where they combine and annihilate each other. During this process, electrons transition from a high-energy state to a low-energy state, releasing the excess energy as light.
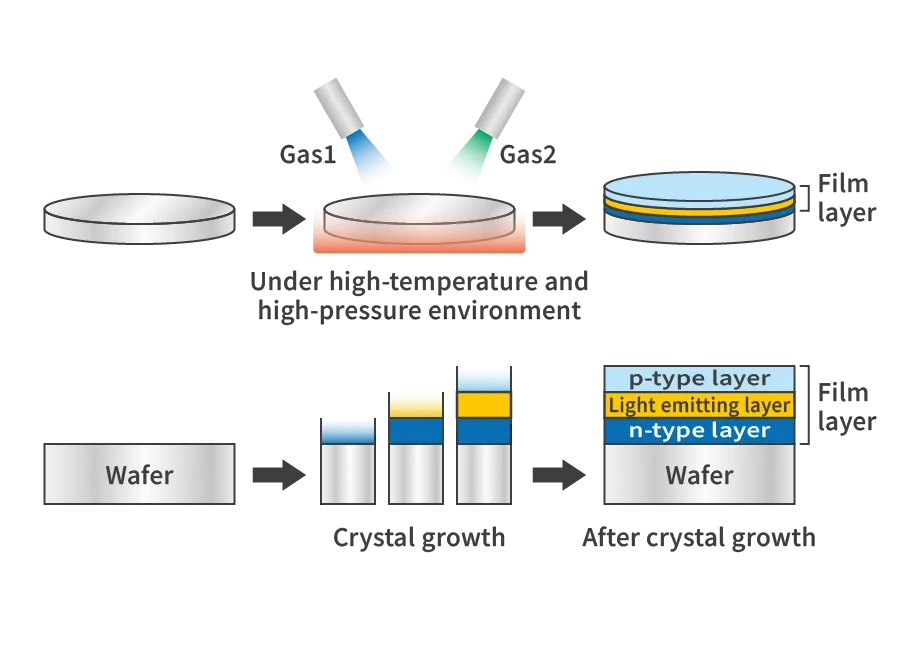
The core of LED manufacturing is “crystal growth.” In this process, a thin film is created on the wafer to emit light as an LED. The top part of the diagram provides an overview of the process, while the bottom part shows a cross-sectional view. The wafer is placed in a high-temperature, high-pressure environment, and gas is sprayed onto it, forming a thin film through a chemical reaction on the wafer’s surface. This process is repeated multiple times to create a multi-layered film structure.
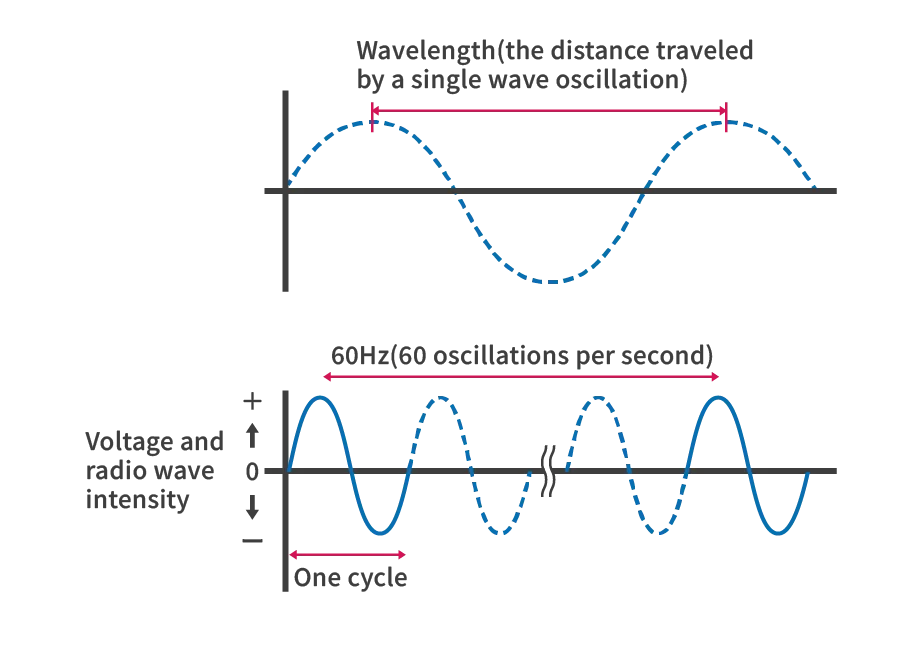
When light behaves as a wave, its motion is represented by a sine curve. The wavelength (symbol λ) is the distance between repeated identical points, such as the highest points (maxima) of the wave, and its unit is nm (nanometers, where 1 nm = 10^-7 cm). The frequency (symbol ν) is the number of repeated waves per unit time and is equivalent to the reciprocal of the period T.
Light is broadly classified according to its wavelength into UV radiation (400 nm or less), visible light (400-800 nm), and IR radiation (800 nm or more). Additionally, there are types of radiation such as alpha rays, beta rays, and gamma rays with wavelengths shorter than UV radiation, and radio waves and microwaves with wavelengths longer than IR radiation.
UV radiation is also electromagnetic waves. The physics of electromagnetic waves is based on quantum mechanics. In quantum mechanics, electromagnetic waves (photons) are neither waves nor particles, but something in between, appearing like waves and like particles depending on the situation—a strange combination of both. Based on this quantum theory, photons can be considered electromagnetic waves in a quantum-mechanically modified sense. (“The Quantum Mechanical World View” by Tomonaga Shinichiro (Kobundo, 1965))
If the energy of one photon is ε, then since light is also a wave, there is a relationship between its wavelength, frequency, and speed of light c:
c=λν
ε=hν=ch/λ
(h is Planck’s constant)
As can be seen from these equations, the energy of light varies depending on the wavelength.
Light in the longer wavelength (lower frequency or wave number) region, such as IR radiation, has less energy, while radiation in the shorter wavelength region, such as UV radiation, has more energy.
“mW/cm²” is the unit of UV radiation dose rate. UV radiation dose rate is also expressed as UV radiation irradiance or UV radiation intensity. This unit is important when selecting UV irradiation equipment. UV radiation dose is calculated by multiplying this UV radiation dose rate by the exposure time (unit: seconds). The formula is:
UV radiation dose (unit: mJ/cm² or mW·s/cm²) = UV radiation dose rate (unit: mW/cm²) × exposure time (unit: seconds)